HIV types 1 and 2 index
Index of pathogens
[Please click on the initial letter of the pathogen or simply scroll down the list!]
Murk JL
The microorganism and its clinical presentation
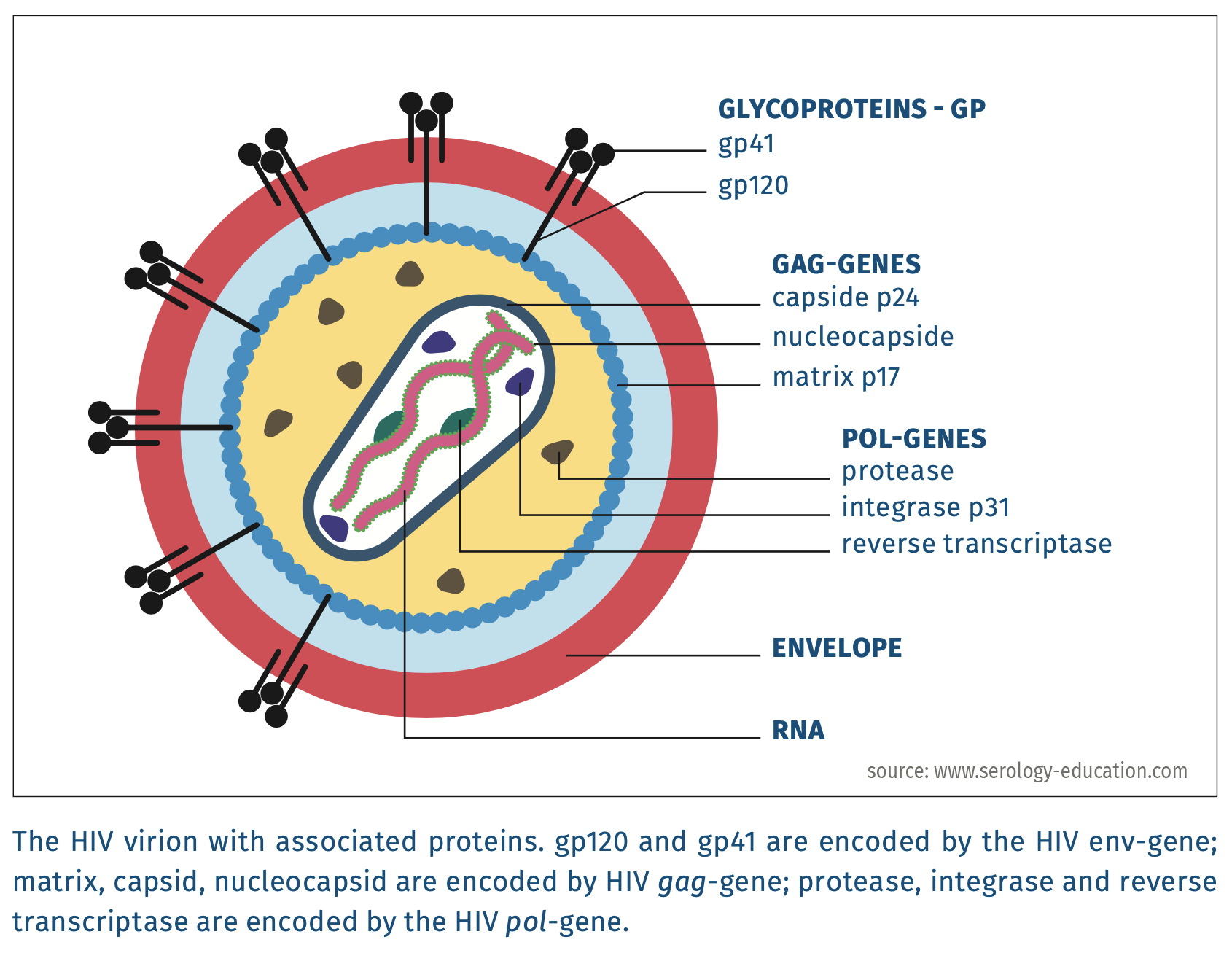
HIV-1 and HIV-2 are enveloped viruses with a diameter of 80-100 nm. They belong to the family of Retroviridae [Int.ComTaxViruses 2019] and contain two homologous, dimerised positive RNA-strands of more than 9 kb [Lentivirus 2019].
HIV causes life-long persisting infections. A primary HIV infection is symptomatic in 50-90% of patients and is referred to as acute retroviral syndrome (ARS) [Richey 2013]. ARS develops 1-6 weeks after exposure (median 3 weeks) and is characterised by mononucleosis like symptoms that are sometimes accompanied by exanthema, neurological symptoms or opportunistic infections. ARS generally resolves within two weeks. Then a symptom free period starts, known as clinical latency, that may last for years or even more than a decade until the patient develops the acquired immunodeficiency syndrome (AIDS). The time to progression to AIDS correlates with many factors including age, the severity of symptoms of ARS, the CD4 count after primary infection and HIV load (set-point) several months after infection in untreated people [Langford 2007].
According to the WHO, HIV infections can be divided into four clinical stages that correlate with CD4+ T-lymphocyte counts [Interim WHO 2005]. In stage 1, there are no clinical signs of immunodeficiency and CD4 counts have normal values (>500 cells/µL). In this stage HIV infection is asymptomatic, although the HIV-infected person can have persistent generalised lymphadenopathy. In stage 2, CD4 counts are between 350 and 500 cells/µL and common infections may occur more frequently. The HIV-infected person may suffer from mild constitutional symptoms, such as weight loss without apparent cause or being more easily fatigued. In stage 3, CD4 counts are between 200 and 350 cells/µL, and signs of immunodeficiency become visible. Common infections have a more severe disease course and the severity of constitutional symptoms also increases. In stage 4, also described as AIDS, CD4 counts are below 200 cells/µL and the person is severely immunocompromised. At this stage the HIV-infected can develop (severe) opportunistic infections, such as esophageal candidiasis and Pneumocystis jirovecii pneumonia, and HIV-associated malignancies, such as Kaposi sarcoma.
Figure 1.
Complications
Untreated HIV infections are generally fatal. Apart from the increased risk for severe infections due to immune dysfunction, HIV infection also increases the risk for malignancies, development of autoimmune diseases [Yen 2017], endocrine and metabolic abnormalities, musculoskeletal and cardiac problems, ocular and neurological disorders, renal disease and haematological disorders affecting all three hematological cell lines [Sterling2014].
Epidemiology
Globally about 42.3 million people are living with HIV [WHO 2024]. HIV is transmitted from person-to-person via sexual contact, blood-blood contact or contact of infected body fluids, such as semen or breastmilk, with blood or mucosal membranes [WHO 2024]. The unborn child can also become infected by placental transmission [Brossard 1995].
Diagnostic testing
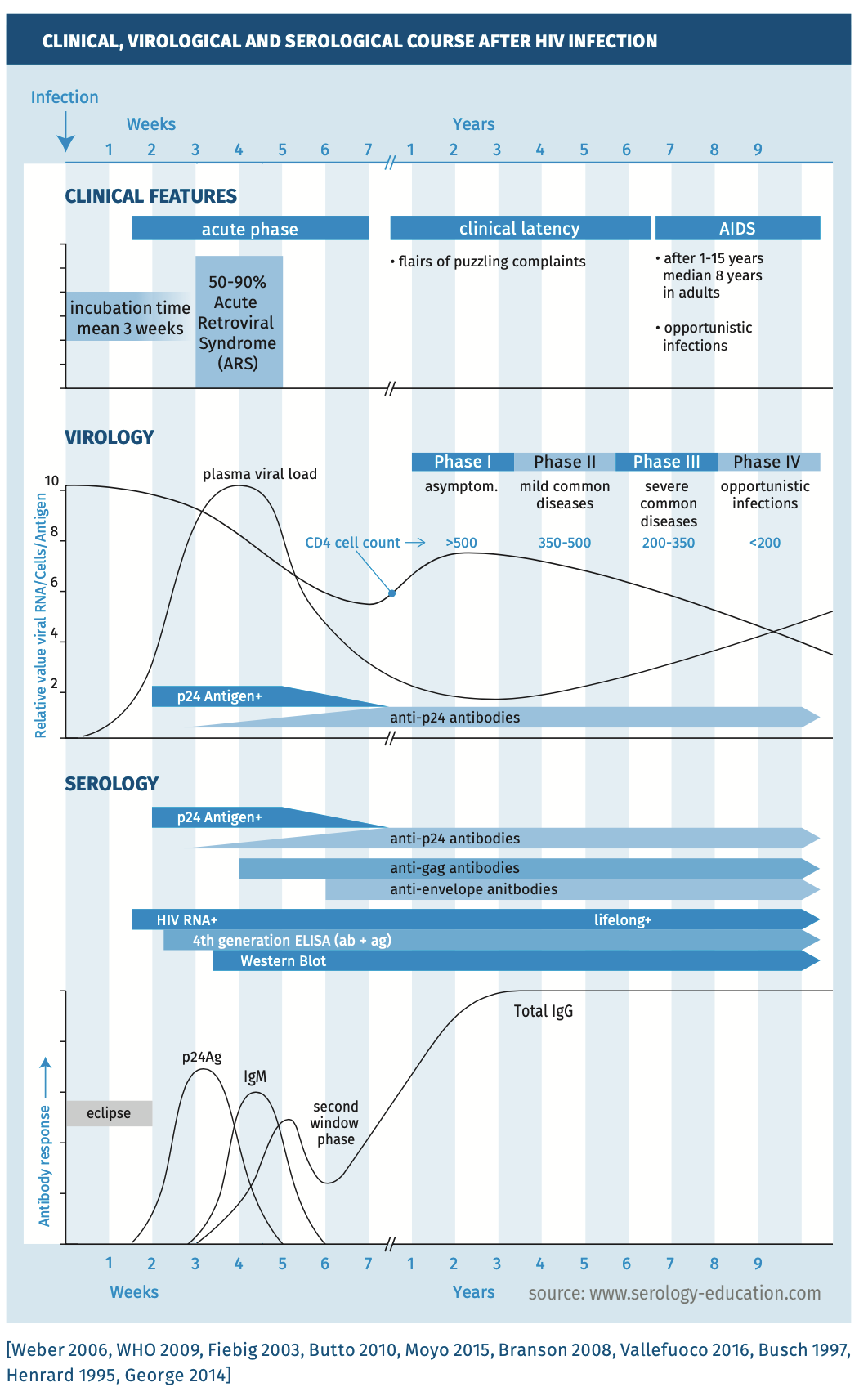
Clinical, virological and serological course is depicted in figure 2: natural course of HIV infection with CD4 counts, HIV RNA, p24 antigen and antibody response and Fiebig staging / classification of HIV infection. Stages are based on the presence of diagnostic markers of HIV infection.
Figure 2.
Natural course of infection
The immunological, virological and serological course is depicted in Figure 2 [Branson 2008, Fiebig 2003].
Techniques
A wide range of diagnostic tests is available to diagnose HIV infection. The assays can be distinguished by the type of target that is detected. So-called molecular tests or nucleic acid tests (NAT) detect (parts of) the genome of HIV (HIV-RNA or proviral HIV-DNA). Serological tests are immunoassays that detect specific antibodies to HIV and/or viral antigen.
- Immunoassays Most laboratories use fourth generation immunoassays which have a superior sensitivity and specificity compared to earlier generations [Vallefuoco 2016]. With each successive generation, the duration of the ‘window period’ - the time between infection and possible detection by a diagnostic test - for HIV diagnosis decreased. The first generation assays utilise HIV-1 lysate derived from infected cell cultures to detect IgG antibodies to HIV. Second generation assays contain synthetic or recombinant HIV antigens to detect IgG antibodies to HIV. First and second generation assays are also called IgG sensitive assays. Third generation assays are sandwich assays that capture specific IgM and IgG antibodies to HIV using recombinant HIV-antigens on the solid phase in combination with conjugated soluble HIV-antigens. These assays are called IgM/IgG sensitive assays. Fourth generation assays combine detection of HIV-p24 antigen with detection of IgM and IgG antibodies as in third generation assays. These assays are also called p24/IgM/IgG sensitive assays. Tests that only detect HIV-p24 antigen are also available.
- Immunoblots Immunoblots contain spatially separated HIV-proteins on a gel or nitrocellulose strip and are used to identify the protein targets of reacting antibodies. Immunoblots are employed to evaluate the specificity of HIV-screening assays and to differentiate between HIV-1 and HIV-2 infections. Immunoblots are less sensitive than fourth-generation immunoassays. They only detect antibodies, including antibodies to p24, but do not detect p24 antigen.
- Antigen The pattern of antibodies to specific HIV proteins, in combination with the presence or absence of p24 antigen, may provide insight about the timing of primary infection (Fiebig staging system [Fiebig 2003], see figure 3). The first serological marker to appear after primary HIV infection is p24 antigen (HIV-1) or p26 antigen (HIV-2); these become detectable from about 15 days after infection [Busch 1997, Henrard 1995]. p24 Antigen usually becomes undetectable within two months, but sometimes even within one or two weeks [George 2014]. Disappearance is likely due to formation of complexes containing HIV-p24 antigen and specific antibodies. It is important to realise that fourth-generation immunoassays do not detect HIV-2 p26-antigen. The diagnostic window for HIV-2 infections is therefore longer than for HIV-1.
- Antibodies Antibodies against HIV that usually appear first are directed against one or more gag-proteins (most notably p24 (HIV-1) or p26 (HIV-2), but also to HIV-1 p17, p55, or to HIV-2 p16, p56); followed by env-gene proteins (for HIV-1: gp41, gp120, gp160, and for HIV-2: gp36, gp105/gp125, gp140, see figures 1 and 2). Antibodies directed against pol-gene proteins (for HIV-1: p31, p51, p66 and for HIV-2: p31/34, p53, p68) usually appear last. The order of appearance of antibodies to these HIV antigens, however, varies from person to person. This variation may have an impact on the duration of the window period. Fourth generation assays generally detect antibodies to env-proteins and pol-proteins, but do not detect antibodies to p24-antigen [George 2014]. In rare cases, a second diagnostic window may develop with fourth generation assays when antibodies to env-proteins appear late [George 2014]. Thus, when p24-antigen becomes undetectable due to formation of p24 antigen-antibody complexes, a positive test may become false negative again [George 2014]. HIV-RNA tests can be used to diagnose HIV during the second diagnostic window.
Practical use of serology
Indications for screening
- After high-risk behavior or possible exposure
- Due to clinical signs / symptoms compatible with HIV infection
- Due to the presence of unexpected infections, malignancies or laboratory findings
- During pregnancy
- Before medical interventions (e.g. transplantation) or before donation of blood, tissue or organs.
Selection of assays to test for HIV
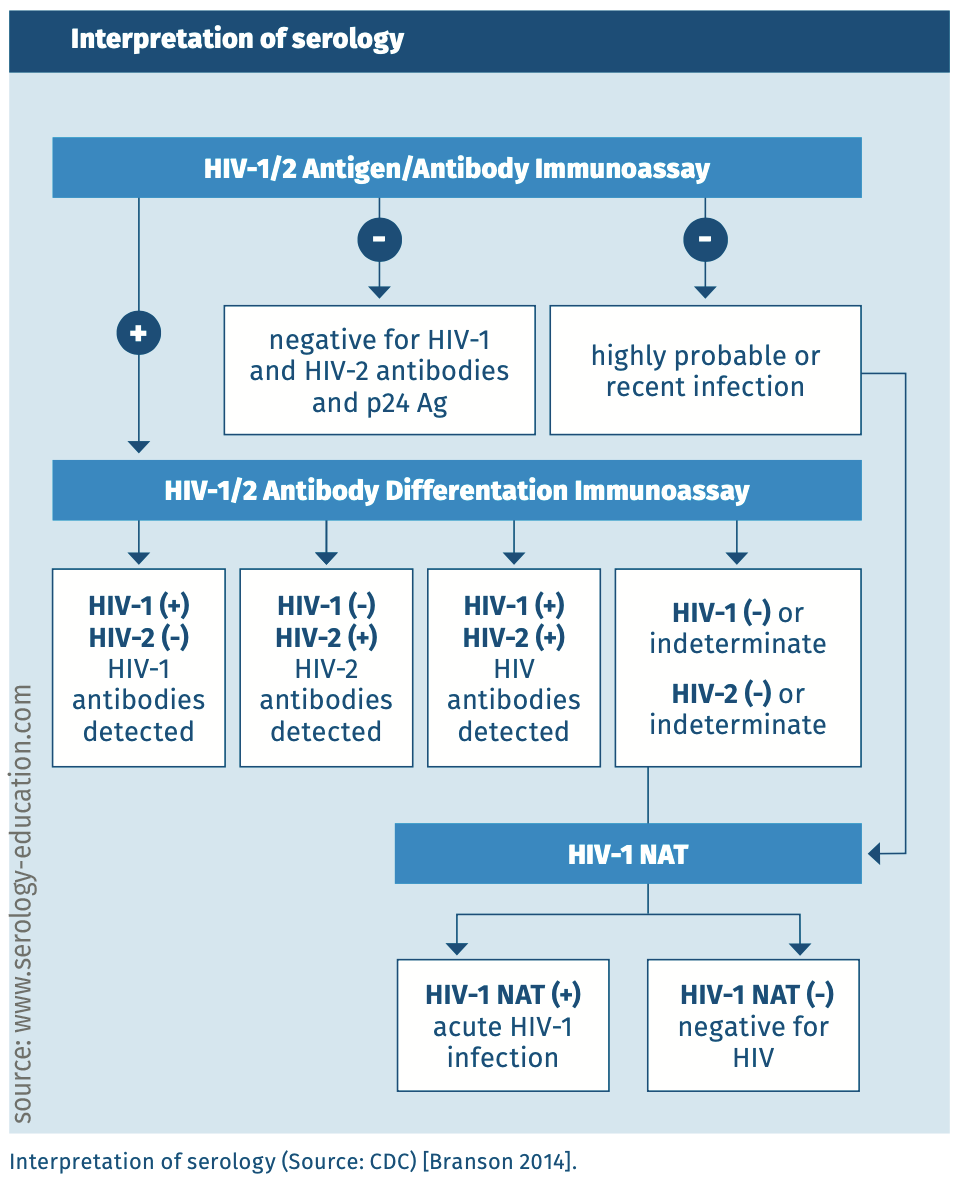
A fourth-generation serological assay should be used, or a third-generation serological assay in combination with a HIV-1 RNA test or separate p24 antigen assay. See figure 3 for the recommended diagnostic algorithm. In specific populations, such as PreP users, a combination of a fourth-generation assay and HIV-1 RNA test may be necessary (see section on different populations). Third and fourth generation assays can detect HIV-1 and HIV-2 infections. Because of the similarities between HIV-1 and HIV-2, antibodies to either one of the HIV-species may cross-react with the other species. Cross-reactivity is the largest for gag and pol-gene products and least for env-gene products (the HIV glycoproteins). However, it is always necessary to use an immunoblot to differentiate between HIV-1 and HIV-2 infection. The HIV-1 RNA test does not detect HIV-2, and it should be noted that a single negative HIV-2 RNA test cannot exclude HIV-2 infection because infected people do not always have viraemia or viral loads may be low [HIV 2019]. An immunoblot should therefore be used to determine the type(s) of HIV infection(s).
Testing in different populations
- Children of HIV positive mothers HIV-infected mothers pass on their IgG antibodies to their children. Traces of these antibodies may remain present for up to 18 months after birth and can neutralise p24 antigen in HIV-1 infected infants [Chantry 1995]. Serology in newborn children is therefore cumbersome. Some centers attempt to test for p24 antigen after using immune complex disrupting (ICD) procedures, which attempt to dissociate (maternal) antibodies from p24 antigen [Gray 2018]. There is no consensus about the reliability of these procedures.
The best way to diagnose vertically transmitted HIV infection is to test for HIV RNA in blood obtained within 48h after birth, preferably before administration of antiretroviral prophylaxis. For up to several weeks after a course of antivirals, a negative HIV RNA test does not exclude HIV infection. Antiretroviral treatment of the mother can also suppress HIV in the unborn child for up to 6 weeks after birth [HIV 2019].
Different guidelines exist to diagnose HIV infection in young children born from HIV infected mothers. It is recommended to consult relevant regional guidelines for diagnostic protocols. - Infected children with early antiretroviral therapy HIV-infected children may not have antibodies to HIV if antiretroviral treatment is initiated during the first months of life. They may also sero-revert due to loss of antibodies to HIV [Alvarez-Uria 2012]. In these cases a negative serological test is not evidence of HIV-cure.
- Primary HIV infection and antiretroviral therapy (failed pre-exposure prophylaxis (PReP) or post-exposure prophylaxis (PEP), initiation of antiretroviral therapy before seroconversion If antiretroviral therapy is started before seroconversion, seroconversion may be delayed or incomplete [Zucker 2018,Donnell 2017]. This can result in false negative serological tests and negative HIV RNA/DNA tests. There is no consensus on how screening should be performed in these situations. In the opinion of this author, HIV-antibody assays (3rd or 4th generation) should always be combined with detection of HIV-RNA, and with repeated testing of follow-up samples. Borderline positive results may be evidence of primary HIV infection.
- Serology after experimental HIV vaccination Persons that participate(d) in HIV vaccine trials may have false-positive or inconclusive HIV serological tests. Most vaccine trails used the envelope protein gp120, but some used gp41/gp160, p24 and/or other HIV proteins. Fourth generation assays are expected to be positive, but immunoblots may yield borderline results.
- Serology after hematological stem-cell transplantation After hematological stem cell transplantation in HIV infected patients, antibody titers to HIV may decrease [Koelsch 2017]. In HIV-cure strategies with CCR5 negative donor cells, HIV antibody titers may decrease or disappear.
INTERPRETATION of SEROLOGY
Figure 3.
SENSITIVITY AND SPECIFICITY
Fourth generation assays have sensitivities and specificities above 98% [Hurt 2017]. It should be noted that during the early phases of HIV infection (eclipse phase) immunoassays are negative. After initially being positive, a second diagnostic window may be present (see section on techniques).
Point-of-care tests for HIV antibodies and/or p24 antigen have lower sensitivities than automated 4th generation assays. Assays that use blood are more sensitive than assays that use saliva. Sensitivities and specificities vary per assay.
Pitfalls
False-negative serology can be caused by:
- laboratory errors,
- inappropriate transport or storage conditions of the sample,
- receipt of antiretroviral therapy at a very early stage of infection,
- sampling during the first or second diagnostic window,
- failure to generate antibodies (and when no p24 antigen test is used). This occurs in a minor proportion of HIV patients and these generally progress rapidly to AIDS (so called rapid progressors) [0xford 2016, Spivak 2010]. Antibody negative HIV infection also occurs in severe hypogammaglobulinemia or severe immune compromise,
- sero-reversion due to early start of antiretroviral therapy, in combination with prolonged antiretroviral therapy or hematological stem cell transplantation [Alvarez-Uria 2012, Koelsch 2017].
False-positive test results occur comparatively frequently, depending on the assay used. False positive tests may be due to:
- laboratory errors,
- in newborns up to 18 months due to maternal antibodies,
- test interference caused by:
- non-specific binding of antibodies related to autoimmune disorders,
- the occurrence of a recent viral infection,
- experimental HIV vaccination,
- presence of drugs or substances in the blood of the patient that interfere with the test.
References
- Alvarez-Uria G, Naik PK, Midde M et al. False negative HIV antibody test in HIV infected children who receive early antiretroviral treatment in a resource-limited setting. Infect Dis Rep 2012;4(1):e6.
- Branson B, McDougal J. Establishing the diagnosis of HIV infection. AIDS therapy.3rd ed.Philadelphia, PA: Churchill-Livingstone and Elsevier Inc 2008:1-22.
- Branson BM, Owen SM, Wesolowski LG et al. Laboratory testing for the diagnosis of HIV infection: updated recommendations. 2014.
- Brossard Y, Aubin JT, Mandelbrot L et al. Frequency of early in utero HIV-1 infection: a blind DNA polymerase chain reaction study on 100 thymuses. AIDS 1995;9(4):359-366.
- Busch MP, Satten GA. Time course of viremia and antibody seroconversion following human immunodeficiency virus exposure. Am J Med 1997;102(5):117-124.
- Butto S, Suligoi B, Fanales-Belasio E. Laboratory diagnostics for HIV infection. Ann Iste Super Santa 2010;vol.46,1:24-33
- Chantry CJ, Cooper ER, Pelton SI et al.. Seroreversion in human immunodeficiency virus-exposed but uninfected infants. Pediatr Infect Dis J 1995 ;14(5):382-387.
- Donnell D, Ramos E, Celum C et al. The effect of oral preexposure prophylaxis on the progression of HIV-1 seroconversion. AIDS 2017;31(14):2007-2016.
- Fiebig EW, Wright DJ, Rawal BD et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 2003;17(13):1871-1879.
- George CR, Robertson PW, Lusk MJ et al. Prolonged second diagnostic window for human immunodeficiency virus type 1 in a fourth-generation immunoassay: are alternative testing strategies required? J Clin Microbiol 2014;52(11):4105-4108.
- Gray ER, Bain R, Varsaneux O et al. p24 revisited: a landscape review of antigen detection for early HIV diagnosis. AIDS 2018;32(15):2089-2102.
- Henrard DR, Daar E, Farzadegan H et al. Virologic and immunologic characterization of symptomatic and asymptomatic primary HIV-1 infection. Journal of acquired immune deficiency syndromes and human retrovirology 1995;9:305-305.
- HIV diagnostic testing - National HIV curriculum. 2019; Available at: https://www.hiv.uw.edu/go/screening-diagnosis/diagnostic-testing/core-concept/all. Accessed 8/8, 2019.
- Hurt CB, Nelson JAE, Hightow-Weidman LB et al. Selecting an HIV Test: A Narrative Review for Clinicians and Researchers. Sex Transm Dis 2017;44(12): 739-746.
- Interim WHO clinical staging of HIV/AIDS and HIV/AIDS case definitions for surveillance. 2005; Available at: https://www.who.int/hiv/pub/guidelines/clinicalstaging.pdf. Accessed 8-5, 2019.
- International Committee on Taxonomy of Viruses. 2019; Available at: https://talk.ictvonline.org/taxonomy/. Accessed 8-5, 2019.
- Koelsch KK, Rasmussen TA, Hey-Nguyen WJ et al. Impact of allogeneic hematopoietic stem cell transplantation on the HIV reservoir and immune response in three HIV infected individuals. J Acquir Immune Defic Syndr 2017;75(3):328.
- Langford SE, Ananworanich J, Cooper DA. Predictors of disease progression in HIV infection: a review. AIDS research and therapy. 2007;4(1):11
- Lentivirus 2019; Available at: https://viralzone.expasy.org/264. Accessed 8-5, 2019.
- Moyo S, Wilkinson E, Novitsky V et al. Identifying recent HIV infections: from serological assays to genomics. Visuses 2015,7: 5508-5524.
- Richey LE, Halperin J. Acute human immunodeficiency virus infection. Am J Med Sci 2013;345(2):136-142.
- Spivak AM, Sydnor ER, Blankson JN et al. Seronegative HIV-1 infection: a review of the literature. AIDS 2010;24(10):1407-1414.
- Sterling TR, Chaisson RE. General clinical manifestations of human immunodeficiency virus infection (Including acute retroviral syndrome and oral, cutaneous, renal, ocular, metabolic, and cardiac diseases). Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases: Elsevier Inc.; 2014. p. 1541. e1-1557. e5.
- Vallefuoco L, Mazzarella C, Portella G. Fourth generation assays for HIV testing. Expert review of molecular diagnostics 2016;16(7):723-732.
- Weber B. Screening of HIV infection: role of molecular and immunological assays. Expert Rev Mol Diagn 2006;6:399-411.
- WHO/Fact sheets/Detail/HIV/AIDS. 2024; Available at: https://www.who.int/news-room/fact-sheets/detail/hiv-aids
- WHO Guidelines for HIV diagnosis and monitoring of antiretroviral therapy. Regional office for South-East Asia 2009.
- Yen YF, Chuang PH, Jen IA et al. Incidence of autoimmune diseases in a nationwide HIV/AIDS patient cohort in Taiwan, 2000-2012. Ann Rheum Dis 2017;76(4):661-665.
- Zucker J, Carnevale C, Rai AJ et al. Positive or Not, That Is the Question: HIV Testing for Individuals on Pre-exposure Prophylaxis. J Acquir Immune Defic Syndr 2018;78(2):e11-e13.
Keywords: HIV, Aids